Narcolepsy New Treatment 2024 – Harmony Biosciences Holdings, Inc. (Nasdaq: HRMY) has announced that the U.S. Food and Drug Administration (FDA) has granted priority review to its supplemental New Drug Application (sNDA) for WAKIX® . The Global Narcolepsy Treatment Industry is on the verge of a substantial breakthrough, with Future Market Insights (FMI) forecasting a remarkable growth trajectory. From an estimated US$4.77 billion .
Narcolepsy New Treatment 2024
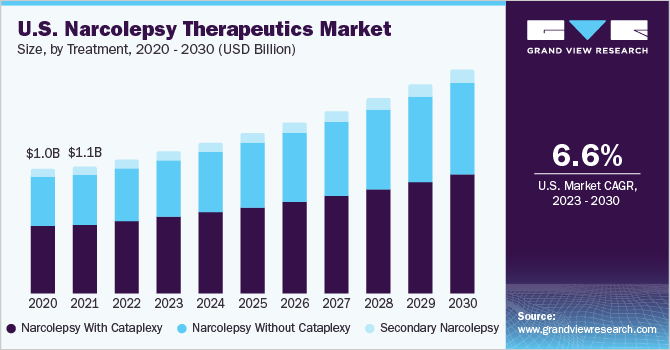
Source : www.linkedin.comNarcolepsy Therapeutics Market Size & Share Report, 2030
Source : www.grandviewresearch.comaxsm ex99_1s19.
Source : www.sec.govSEC Filing | Alkermes plc
Source : investor.alkermes.com$18B+ AbbVie Acquisitions & Partnerships, Amylyx CMO Announced
Source : www.linkedin.comZenzedi: ADHD medication recalled due to pill mixup | CNN
Source : www.cnn.comAdvances in Narcolepsy Treatment | MyNarcolepsyTeam
Source : www.mynarcolepsyteam.comTreatments for Narcolepsy | MyNarcolepsyTeam
Source : www.mynarcolepsyteam.comRegistration for 2024 Wake Up Narcolepsy National Summit is NOW
Source : www.wakeupnarcolepsy.orgJulie Flygare
Source : www.facebook.comNarcolepsy New Treatment 2024 Global Narcolepsy Treatment Market 2024 2030 Share & Upcoming Trend: Harmony Biosciences Holdings, Inc. (Nasdaq: HRMY) announced that the U.S. Food and Drug Administration (FDA) granted priority review for its supplemental New Drug Application (sNDA) for WAKIX® . InvestorPlace – Stock Market News, Stock Advice & Trading Tips The biotech sector is in the spotlight in 2024, where under-the-radar biotech .
]]>